OUR MISSION
BlueSky Immunotherapies GmbH (Ltd.) is a clinical-stage biotech company. The company’s business is based on its own proprietary, novel technology platform for interferon-inducing viral vectors (delNS). Interferons have immunostimulatory and antiviral properties. BlueSky uses these delNS- mediated properties for therapies against cancer and viral infectious diseases.
TECHNOLOGY
The high level of interferon induced by the delNS vector activates immunological defence reactions against cancer. It also stimulates the production of antiviral molecules:
Immunostimulation / Immunomodulation
– NK –
activates Natural Killer Cells
– CTL –
activates Cytotoxic T-Cells
– DC –
activates Dendritic Cells
– Treg –
inhibits Immunosuppressive Regulatory T-Cells
– ΜΦ –
activates Macrophage
Antiviral molecules / Inhibition of viral replication
– ISG15 –
Interferon stimulated gene 15
– OAS –
Oligoadenylate synthetase
– PKR –
Protein Kinase R
– Mx –
Interferon-induced GTP binding protein
REFERENCES / PUBLICATIONS delNS
The original paper Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems (Virology 252,324) has accumulated 1248 citations in peer – reviewed journals (Google Scholar, December 2021).
The immunogenic properties and safety of delNS-based viruses have been widely published in top journals. A selection of corresponding papers is provided below:
- PLoS ONE 16(11): e0260155 / Elimination of equine sarcoids (33804 downloads)
- Scientific Reports 11, 22164 /Antiviral properties through interference (35732 downloads)
- J Virol 74,6203 / activation of PKR (32361 downloads)
- J Virol 76,1617 / induction of apoptosis (32050 downloads)
- J Infect Dis 201,354 / B-cell mediated local and systemic response (33119 downloads)
- Med Microbiol Immunol 199,93 / activation of NK cells (33130 downloads)
- Vaccine 37,3722 / B-cell mediated response (32740 downloads)
- PLoS One 10(9) e0138722 / T-cell mediated response against E6E7 (31981 downloads)
- Nature 424,324 / activation dentritic cells and induction IFN (31755 downloads)
PIPELINE OVERVIEW
The pipeline spans HPV-induced indications already in clinical development, as well as rapidly advancing preclinical programs targeting breast and prostate cancers.
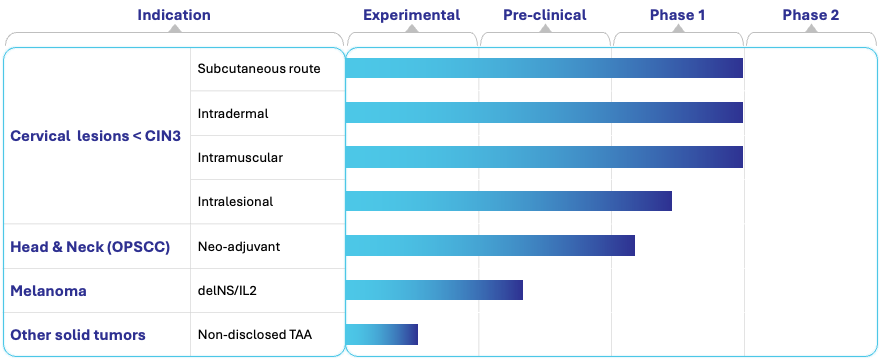
PIPELINE DESCRIPTION
1. HPV-Related Diseases (Clinical Stage)
HPV-Induced Cervical Lesions (<CIN3)
Vector: delNS/E6E7
Status: Phase 1 systemic routes (s.c., i.d., i.m.) completed, intralesionsal ongoing. Phase 2 planned
Completed Phase 1 study demonstrated an excellent safety profile and >80% elimination of precancerous lesions, along with clearance of human papillomavirus (HPV)—confirming the platform’s unique dual mechanism in humans.
Cervical Cancer (HPV-driven)
Vector: delNS/E6E7
Status: Advancing to Phase 2
Building on early efficacy, upcoming trials will assess tumor regression, durability, and HPV eradication in higher-grade cervical disease.
HPV-Positive Head and Neck Cancer (OPSCC)
Vector: delNS/E6E7
Status: Phase 1 ongoing
Interim neoadjuvant data demonstrate strong infiltration of CD8⁺, CD4⁺ T cells and macrophages, and PD-L1 upregulation, confirming potent tumoral immune activation. These results support combination strategies with PD-1/PD-L1 inhibitors.
2. Cross-Species Mechanistic Validation
Equine Sarcoids (Papillomavirus-Induced Tumors)
Vector: delNS/E6E7
Status: Ongoing clinical program
BlueSky has shown complete elimination of aggressive and otherwise incurable papillomavirus-induced tumors in horses, offering compelling cross-species mechanistic validation and demonstrating broad applicability to papillomavirus-driven disease.
3. Expanded Oncology Programs (Preclinical)
Breast Cancer
Vector: delNS expressing breast tumor antigens
Status: Preclinical
Building on the platform’s validated mechanism, BlueSky is advancing antigen constructs targeting breast cancer–associated epitopes.
Prostate Cancer
Vector: delNS expressing prostate tumor antigens
Status: Preclinical
The program focuses on antigens relevant to aggressive and castration-resistant prostate cancer, leveraging the delNS platform’s ability to reshape the tumor microenvironment via strong interferon induction.
Summary
The delNS platform is now backed by strong human clinical validation, demonstrating lesion elimination, HPV clearance, and tumor immune activation. These findings support BlueSky’s strategy to expand beyond HPV-related disease toward solid tumors including breast and prostate cancer, while also advancing combination immunotherapy strategies.
OUR TEAM
Thomas Muster
– CEO –

Michael Tscheppe
– CFO –

Christina Nicolodi
– Chief Regulatory Officer –

Markus Wolschek
– CSO –

ADVISORY BOARD

Noel Barrett, Ph.D.
– Board Member –
- 40+ years in Pharma/Biotech: Extensive management experience in R&D for innovative products
- Former Global VP R&D at Baxter Healthcare: Led the vaccines division
- Former CEO of abiotech start-up specialized in developing immunotherapies for neurodegenerative and cardiovascular diseases

Katherine Cohen, Ph.D.
– Board Member –
- 25+ years in Biotech industry: strategic advisor, venture capital investor, biotech entrepreneur and executive
- Former venture partner at Panacea Venture, former CEO at Hookipa Biotech (Hookipa Pharma) and former SVP at Intercell (Valneva)
- European Patent Attorney and Honorary Professor – University of Applied Sciences Austria

Rüdiger Herrmann, Ph.D.
– Board Member –
- Experienced international lawyer specialized in M&A and licensing, experienced in life science matters in Europe, China, and Taiwan
- Partner at SquirePatton LLP – specialized in biotechnology and pharmaceutical sectors
- Highly Ranked Attorney – recognized in directories such as JUVE, Chambers Europe, and Legal 500

Josef Poandl
– Board Member –
- Experienced business angel in diverse industry, 30+ years senior roles in local and global companies
- Co-founding and leading AmRest Serbia., co-founder and investor in Ecko, BlueSky Immunotherapies GmbH, Nuvonis Technologies GmbH
- Property development and management: Managing partner at ProFood Invest GmbH since 2007 and involved with STELLAR Immobilienverwaltung GmbH since 2000